×
The Standard e-Paper
Fearless, Trusted News
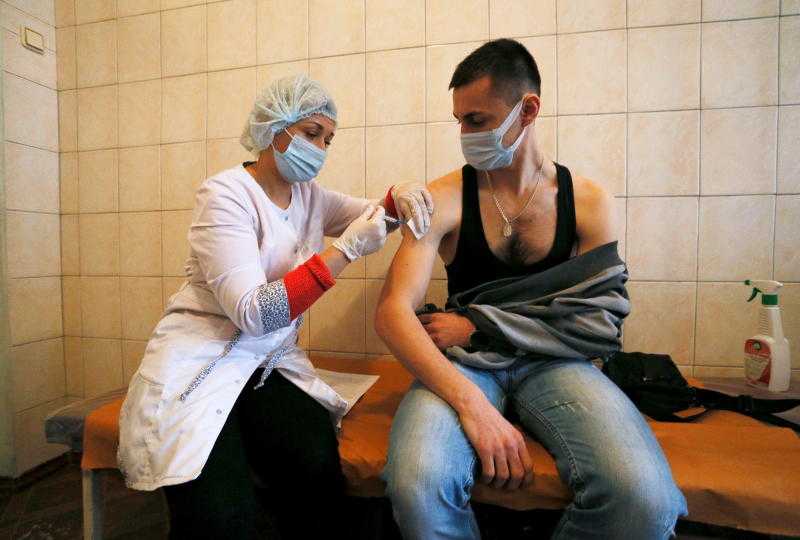
A man is injected with Sputnik-V vaccine against the coronavirus disease in Ukraine on February 3, 2021. The vaccine is soon going to be available in Kenya through a private pharmacy. [Reuters]
The Pharmacy and Poisons Board (PPB) has given Emergency Use Approval for the Russian-developed Covid-19 vaccine, Sputnik V, in Kenya through a private pharmacy.