Pharmacy and Poisons Board issues warning about counterfeit Ozempic Pens.
The Pharmacy and Poisons Board (PPB) has warned the public against using Ozempic Pens, also known as Semaglutide.
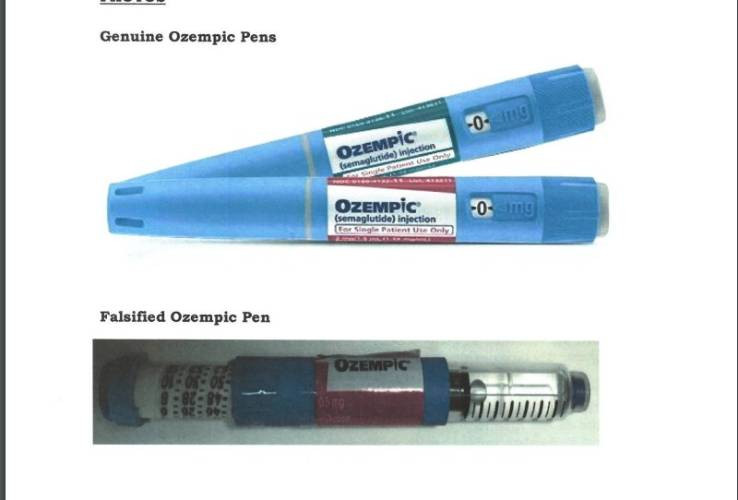
In a statement on Thursday, July 18, PPB noted it has not authorised or registered any Ozempic Pens for use in the Kenyan market.
According to the Board, healthcare providers are prescribing Apidra Solostar Pens, which are intended for treating type 1 and 2 diabetes and relabelling them as Ozempic Pens.
"The Pharmacy and Poisons Board wishes to draw the attention of the public to an alert concerning the falsification of Ozempic Pens where Apidra Solostar Pens have been relabelled as Ozempic Pens," read the statement.
Scientifically, Ozempic Pens are used to lower blood sugar levels by helping the pancreas produce more insulin. They are primarily used as a treatment for type 2 diabetes.
However, some individuals use them for weight loss, despite not being approved for this purpose.
The Board has expressed concern that using falsified Ozempic Pens could jeopardise public health and safety.
- Kenya recalls Benylin cough syrup over safety concerns
- Be wary of fake cancer drug in the market, Poisons Board warns
- Pharmacy Board recalls Allied Limited's nasal drops, Efinox
- Pharmacy and Poisons Board recalls cancer drug over safety
Keep Reading
PPB urged Kenyans to exercise caution and avoid trading or administering these pens until proper surveillance of the product currently in the market is completed.
 The Standard Group Plc is a multi-media organization with investments in media platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national and international interest.
The Standard Group Plc is a multi-media organization with investments in media platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national and international interest.