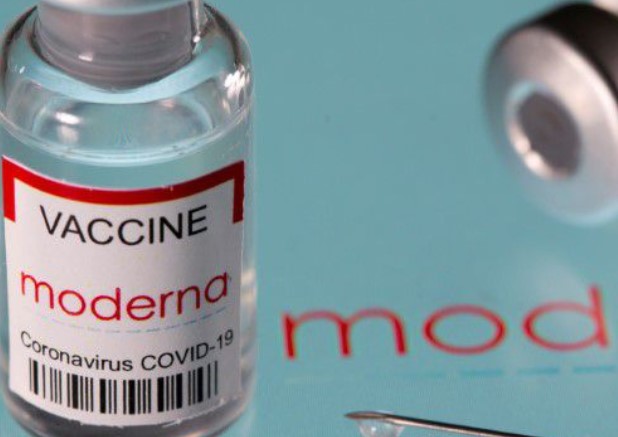
Vial labelled "Moderna coronavirus disease (COVID-19) vaccine" placed on displayed Moderna logo is seen in this illustration picture. [Reuters]
Japan's COVID-19 vaccination push suffered a blow from widening reports of contamination in supplies of Moderna Inc's (MRNA.O) vaccine.
Here are key aspects of the concerns.
WHEN WAS IT FIRST REPORTED AND HOW HAS IT WIDENED?
The first contamination was reported to Moderna's local distributor, Takeda Pharmaceutical Co (4502.T), on Aug. 16. That led to the discovery of 39 vials containing foreign material, all from the same lot of 57,000, or about 570,000 doses.
On Aug. 26, Japan said that, as a precaution, it had halted use of 1.63 million Moderna doses shipped to 863 vaccination centres nationwide.
It suspended the use of lot 3004667 that included those vials and two similarly sized lots - 3004734 and 3004956.
On Sunday, a vaccination centre in Okinawa prefecture reported finding contaminants from a fourth lot, 3005293.
Black substances were spotted in syringes and a vial, with pink substances found in another syringe, the health ministry said.
A vaccine centre in Gunma prefecture near Tokyo found a tiny, black substance in a vial from lot 3005236.
Regional authorities temporarily halted use of the affected lots from Okinawa and Gunma. Later, the health ministry and Takeda said there were no safety concerns and no suspension of use was necessary.
The Okinawa cases were probably caused by needles being incorrectly inserted into vials, breaking off bits of the rubber stopper, Health Minister Norihisa Tamura told reporters on Tuesday.
WHAT ARE THE CONTAMINANTS?
The contaminants that triggered suspensions of the first three lots are believed to be metallic particles, public broadcaster NHK said, citing health ministry sources.
The ministry has not officially identified the particles, pending an investigation by Takeda, Moderna, and its Spanish contract manufacturing partner Rovi (ROVI.MC). The firms have also yet to comment on what the foreign material is.
IS THERE A SAFETY ISSUE?
Japan and Moderna have said no safety issues were identified.
Two men, aged 38 and 30, died this month within days of receiving their second Moderna doses, the health ministry said on Saturday. Each had a dose from one of the suspended lots, 3004734. The causes of death are being investigated.
Each had a fever the day after his second dose and died two days after getting the fever. There has been no evidence of contaminants in their shots, a health ministry official told reporters.
By Aug. 8, 991 people had died in Japan after receiving COVID-19 shots from Pfizer (PFE.N)/BioNTech, and 11 after receiving Moderna's. No causality has been established between the injections and deaths, according to the health ministry.
About 500,000 people have had shots from the first three suspended batches, Taro Kono, the minister in charge of the vaccine push, told reporters on Friday.
ARE OTHER COUNTRIES ALSO AFFECTED?
Rovi, which packages Moderna vaccines for markets other than the United States, said last week the batch in question was meant for distribution only in Japan.
South Korea, which also receives the Moderna vaccines produced from sites in the United States and Spain, said it was not affected.
Last week, the European Union drugs regulator said it was investigating the incident but an initial assessment yielded no reason to seek a temporary suspension of production.
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.