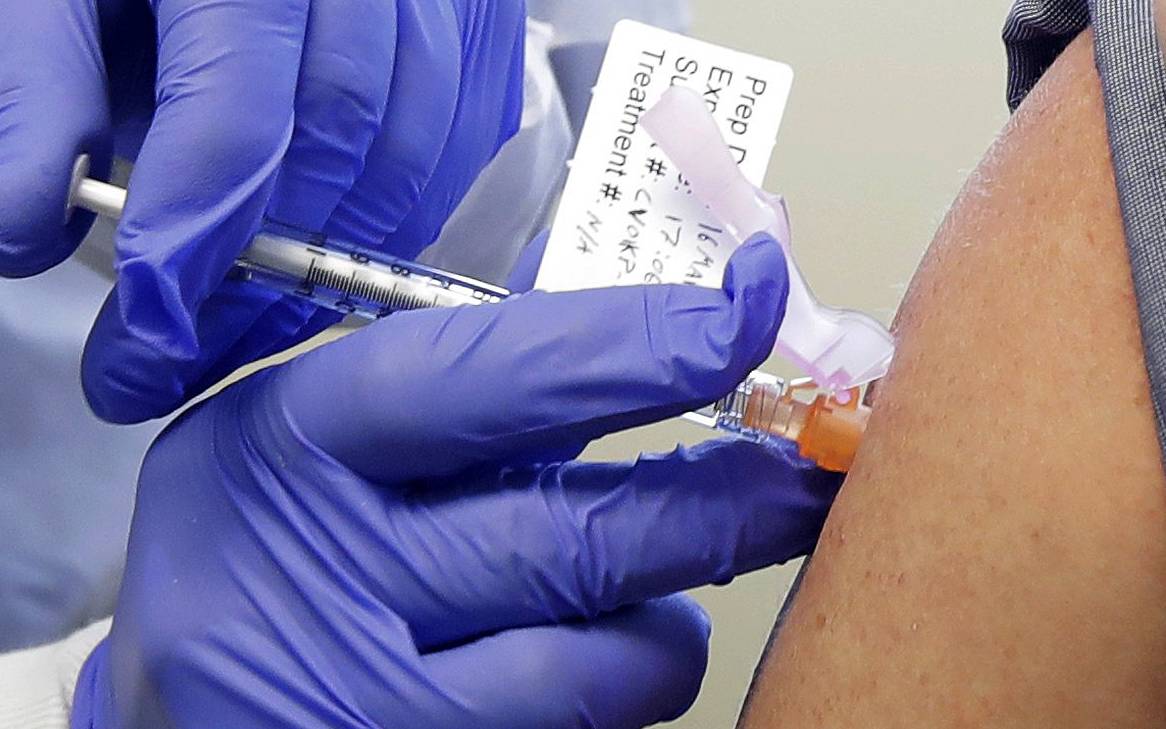
Drug developer Moderna Inc said on Monday it has dosed the first patient with its experimental coronavirus vaccine in an early-stage trial being conducted by the U.S. National Institutes of Health.
Moderna’s study has made the most headway among efforts by the pharmaceutical industry to develop coronavirus vaccines as it is the first to be tested in human patients.
Its vaccine uses synthetic messenger RNA (mRNA) to inoculate against the coronavirus. Such treatments help the body immunize against a virus and can potentially be developed and manufactured more quickly than traditional vaccines.
Moderna joins BioNTech SE, a German drug developer working on mRNA-based treatments, which entered into a partnership on Monday with Shanghai Fosun Pharmaceutical to develop and distribute a coronavirus vaccine in China.
BioNTech said it was in advanced talks with its existing partner Pfizer to develop the vaccine outside China.
Another German drugmaker, CureVac, aims to be ready by July to request permission for human testing of its experimental vaccine, while Johnson & Johnson has said it is optimistic it can start vaccine testing later this year.
German officials are trying to stop the U.S. government from persuading CureVac to move its research to the United States, with German politicians insisting that no country should have a monopoly on any future vaccine, Reuters reported on Sunday.
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.