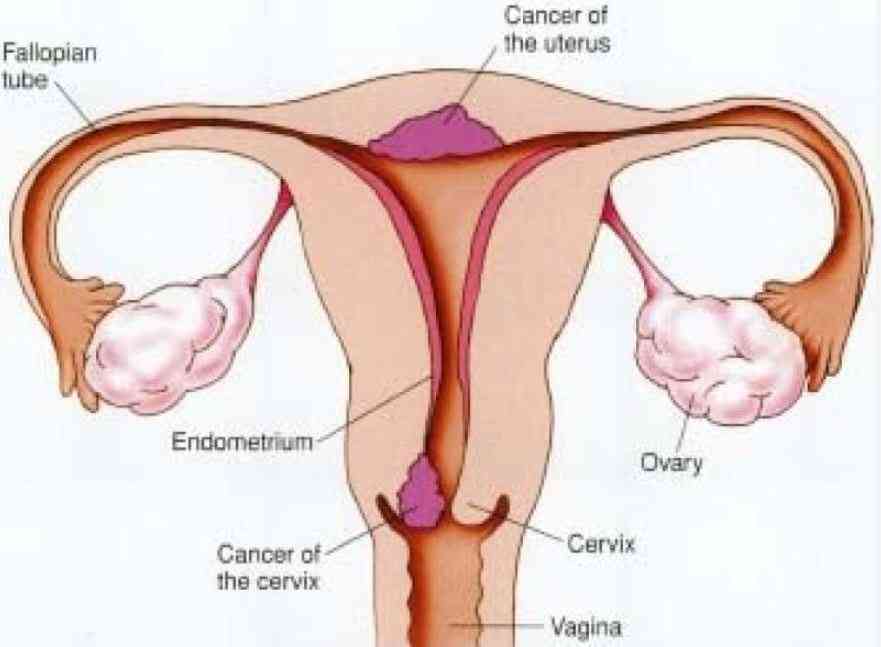
Kenya has no ownership claim to a breakthrough cervical cancer treatment developed at Kenyatta National Hospital three years ago, it has emerged.
A British couple has laid claim to the invention, University of Manchester, UK, the patent and a New Zealand firm, the manufacturing rights.
“Kenyans have no ownership claim in any of this. They did not invent anything, do not co-own the patent while future research and manufacture is the business of Douglas Pharmaceuticals of NZ,” says Dr Rich Ferrie of University of Manchester (UoM).
But Kenyatta National Hospital deputy head of reproductive health Dr Innocent Maranga, under whose watch the study was carried out, says they will not take this new lying down.
“We are in the process of applying for a review of the patent with a view of being enjoined,” says Dr Maranga.
The breakthrough, which had created a buzz globally, was announced, on 6 March 2014 by KNH Chief Executive Officer, Lily Koros.
KNH researchers, working with the UK had developed highly effective, easy to administer and inexpensive treatment for early cervical cancer.
Done deal
Back then, this celebratory mood was capped by even better news from one of the local investigators Prof Peter Gichangi of the University of Nairobi.
“We are in this together with the UK. In fact, KNH and the University of Nairobi are enjoined patenting the treatment. We have documentation with us today to prove this,” he was quoted saying.
However, Prof Gichangi on Thursday said they did not have such documents but had been working on the assumption that this was a done deal.
The study which involved — Drs Ian and Lynn Hampson (a UK coupple) at the UoM had screened 820 women attending reproductive health clinic at KNH settling on 40 who had signs of early cervical cancer.
When put on the experimental treatment, it was found that the drug had wiped out pre-cancerous cells in 90 per cent of trial participants with minimal side effects. This was a major development.
The next level was to move to Phase II trials which, the researchers said would involve about 5,000 pre-cancerous women in Nairobi.
But before this could happen, the study first had to be published in a peer reviewed scientific publication. This happened in 29 January 2016 in the journal Plos One.
And that is when the first signs of trouble started. In the publication, the University of Manchester was indicated to have applied for an EU patent on 23 October, 2014 as sole owners of the intellectual property rights.
The patent application, had Drs Ian and Lynn Hampson as sole inventors and no mention of the Kenyan scientists, KNH or even UoN.
When The Standard on Sunday made inquiries on this, Dr Ian Hampson referred the team to Dr Rich Ferrie the director of Intellectual Property Ltd at UoM.
“There must be an unfortunate misunderstanding. The Intellectual Property is the sole property of the University of Manchester,” wrote Dr Ferrie.
“In essence, the Kenyan team tested an invention already conceived by the Manchester team. Ownership of the patent therefore belongs to the University of Manchester.”
In an interview, Dr Maranga, and Prof Gichangi stated that local researchers had been crucial to the invention. Dr Maranga said he had been assured by the head of the hospital’s legal department, Mr Calvin Nyachoti that KNH is going to appeal the patent exclusion.
Lucrative rollout
But this may be too little too late. About 13 days ago, UoM announced entering into an agreement with the New Zealand firm Douglas Pharmaceuticals for more research and manufacturer of the treatment.
This week, New Zealand media reported that Douglas Pharmaceuticals will build on the contributions made by the Kenyan women and sponsor Phase II studies in the UK and elsewhere in Europe.
Douglas chief executive officer, Jeff Douglas said they have been working with the University of Manchester for two years now and if clinical trials in the Americas and European centers are successful then a potentially hugely lucrative rollout would be made globally.
But there is nothing for Kenya in the UoM/Douglas agreement.
Ironically Douglas and the Hampsons are only able to take the final human research trials to the UK and Europe because Kenya had allowed its women to risk an untested treatment.
In earlier reports in the UK’s Telegraph, it was said that the clinical trials found their way to KNH because it would have been almost impossible to apply an untested treatment on human subjects in the UK.
Then, Dr Maranga a PhD student at UoM, working closely with the Hampsons had consequently introduced the project to KNH.
We did put these ethical questions to the KNH/UoN Ethical Board in writing, but we are yet to get any feedback.
But more important we also wanted to understand project funding.
The study in question is registered as ISRCTN48776874 DOI 10.1186/ISRCTN48776874 and lists the charitable organisation Cancer Research Trust Kenya as one of the funding agents.
Interestingly, the Trust, which has Dr Maranga as its chairman was registered in Kenya five months into the trials. However, as would have been expected this aspect was not declared in the final study publication.
“I don’t consider this as a case of conflict of interest because the Trust was not a donor as erroneously indicated in the study registry,” Dr Maranga explained.
All this basically means that local participants: KNH, UoN, local researchers and women subjects will not benefit from expected huge financial royalties from the resultant product. It also means in future Kenya, like anybody else will have to import the treatment for its women and girls at commercial rates.
The next phase of the study would involve about 5,000 pre-cancerous women with the obvious benefits of possible cure.
www.rocketscience.co.ke
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.