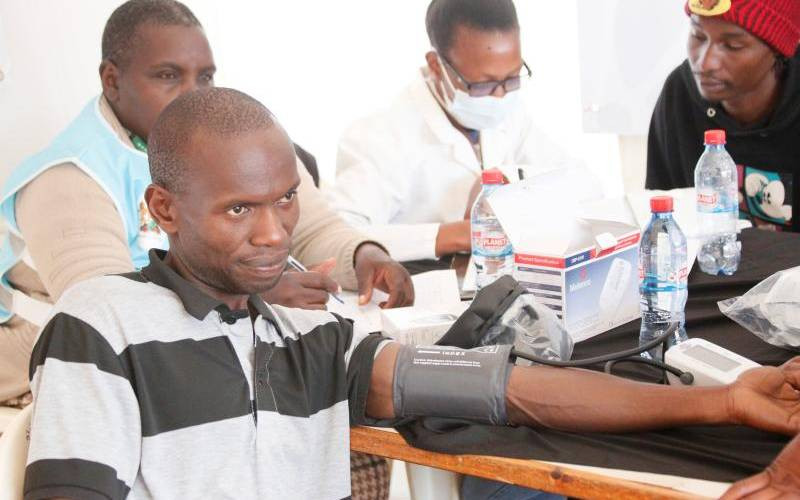
Four hundred health workers from Kilifi and Mombasa will be recruited to participate in a Covid-19 vaccine trial.
The trial, also being tested in the UK by the University of Oxford, are planned to start at the end of this week and will be conducted by the Kenya Medical Research Institute (Kemri)-Wellcome Trust Research Programme that is based in Kilifi at the Kenyan Coast.
It will take two years (until May 2022) and is being sponsored by the University of Oxford and funded by the UK charity, Wellcome Trust, which is highly- influential within the Kenya Medical Research Institute (Kemri).
The trial will be conducted at Kemri’s Kilifi centre, though the study protocol indicates more sites may be opened as the need arises.
The trial registration, number PACTR202005681895696 with the World Health Organisation (WHO), will determine "if a new Covid-19 vaccine safely generates protection in adults in Kenya."
The protocol says while there are many Covid-19 vaccine candidates in development, none of these are in development in Africa and no vaccine trials are currently ongoing in the continent.
“Vaccine performance from studies in other populations may not be generalisable to Kenya and other African countries,” says the protocol.
The trial will evaluate the safety and protectiveness of the vaccine called ChAdOx1 nCoV-19 among 400 adults aged over 18 years.
The trials will take place in the coastal counties of Kilifi and Mombasa, “where high numbers of Covid-19 cases have been reported.”
The study will only enroll healthcare workers, including doctors, nurses, clinical officers, pharmacists, mortuary attendants and allied healthcare professionals.
“Health workers have been prioritised due to their high risk of occupational exposure to Covid-19, hence their urgent need for protection,” says the protocol.
Responding to inquiries, Kemri cited the call by WHO to African countries to be part of an effort to find “One Vaccine for all” to support the fight against Covid-19 for the trials planned in Kilifi.
“Kemri has plans to be part of this global race to find an effective vaccine by trialing the ChAdOx1 nCoV-19 in Kilifi,” Kemri said in an email to The Standard yesterday.
It, however, added that like every trial there were regulatory requirements that must be met at the national and institutional levels. “We would like to emphasise that Kemri is not yet at the stage of implementing this trial. The regulatory process includes review and approval by National Commission for Science, Technology and Innovation, the Pharmacy and Poisons Board, the internal Kemri scientific and ethics review committee, among others,” read the statement.
It added: “Please note that the regulatory process also requires that the trial team does not engage with any stakeholders until the review and approval process are complete. It is only after the process of approval and review that the trial team will be allowed to proceed with the trial and engagement. The team also plans to engage with the various stakeholders involved in this process; this includes the prospective participants groups, especially the health professional bodies, the public, to ensure understanding of the process, and allowing for informed consent. We would urge objective reporting and coverage of this work, especially in this infodemic age.”
The registration of the local vaccine trial over the weekend saw Kenya, for the first time, appear in the global WHO daily tracker of ongoing Covid-19 vaccines and treatment trials.
By yesterday there were 941 such trials going on across the world, USA leading with 167, followed by China with 164. Other countries with many ongoing trials include Iran, 99, Spain 86, and 82 in France.
With the listing, Kenya becomes the first and only country with a Covid-19 vaccine or drug trial in East and Central Africa. South Africa, in the Sub-Sahara region, leads with six trials, Nigeria and Ghana two each, while Senegal has one.
Egypt, with 22 trials, is the highest in the continent, but none of the African countries has a home-developed product, all being collaborations mainly with Europe and Asia.
The start date for the Kenya trials indicated as May 31 will be confirmed by an ethical approval from Kemri. The first phase is scheduled to take one year.
Participants must be healthy adults aged 18 to 55 - both male and females. However, females should not be breastfeeding or pregnant and should not be willing to conceive within the study period.
Females willing to participate must use an effective contraceptive method 30 days prior to vaccination and throughout the study duration.
“For female participants, we will ask them to attend with their family planning records for verification,” says the protocol.
Examples of an effective contraceptive includes a sterilized male partner at least six months prior to the female subject’s entry into the study, and this must be a monogamous relationship.
The trials will be led by Prof George Warimwe of the Wellcome Trust programme, who has been involved in the development of vaccines against the Rift Valley Fever and the Middle East Respiratory Syndrome (MERS).
Human trials for the Covid-19 vaccine that is to be tested in Kenya started last month in the UK, where 800 volunteers will be vaccinated in the initial phase.
The Coronavirus has affected more than 5 million people globally, with at least 340,000 deaths, and was declared a pandemic by WHO. Currently there is no known treatment of Covid-19, but there are several prevention and containment measures implemented by nations, including lockdowns, restrictions in travels, social distancing and hand washing.
“As the cases continue to rise globally there is consensus by scientists that in addition to effective treatments, an effective vaccine remains the most effective way to control the Covid-19 pandemic. Several vaccine candidates are in development, including in Africa, and Kemri is at the forefront of this fight with the development of its own vaccine candidate. There are various vaccine candidates currently in trials across the world. They include the ChAdOx1 nCoV-19 in the UK. However, Immunogenicity and safety data from studies in other populations may not be generalisable to Kenya and other African countries,” added Kemri statement.
 The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.
The Standard Group Plc is a multi-media organization with investments in media
platforms spanning newspaper print
operations, television, radio broadcasting, digital and online services. The
Standard Group is recognized as a
leading multi-media house in Kenya with a key influence in matters of national
and international interest.