×
The Standard e-Paper
Smart Minds Choose Us
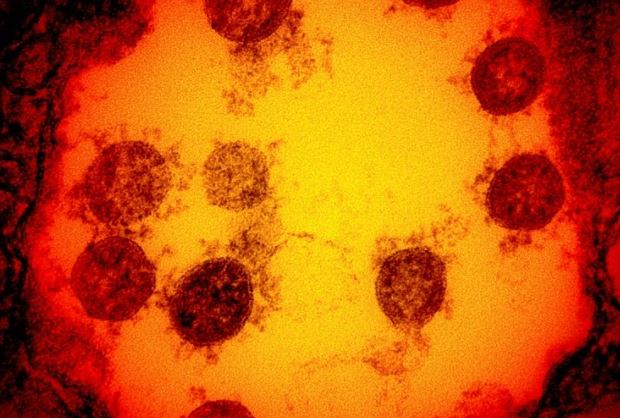
Two late-stage Covid-19 medical trials have been paused in the space of 24 hours over potential safety concerns, the latest setbacks to scientists in the long fight against the pandemic.