×
The Standard e-Paper
Home To Bold Columnists
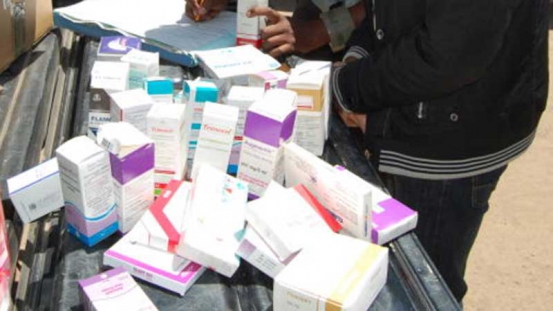
The generic drugs you bought from the pharmacy are probably to blame for that sore throat that just won’t go away no matter how much medicine you take.
It has emerged that more than a dozen pneumonia generic drugs from Nairobi pharmacies have failed to meet internationally accepted benchmarks at the National Quality Control Laboratory (NQCL).