×
The Standard e-Paper
Home To Bold Columnists
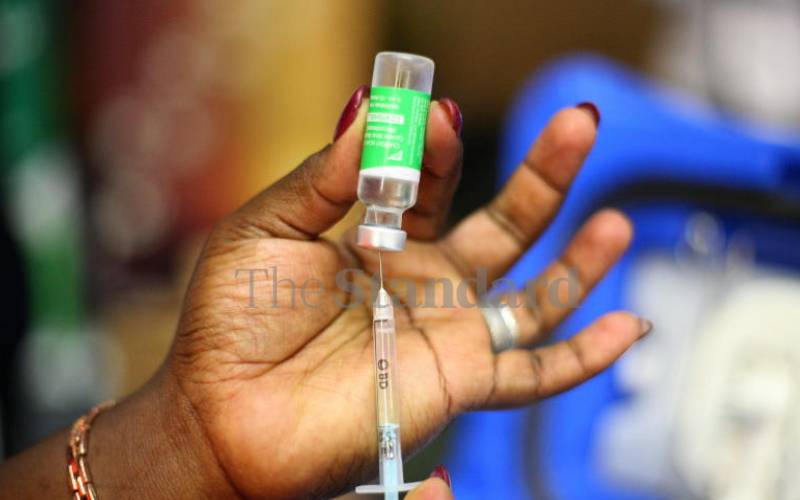
A nurse prepares the Oxford/AstraZeneca vaccine in Nairobi on April 21, 2021. [Wilberforce Okwiri, Standard]
Kenya's biggest economic and health challenge, the Covid-19 pandemic, has been allocated Sh15.4 billion of which Sh3.9 billion will be for procuring vaccines and Sh9.5 billion for engagement of specialists, tests and supplying equipment to hospitals.