×
The Standard e-Paper
Home To Bold Columnists
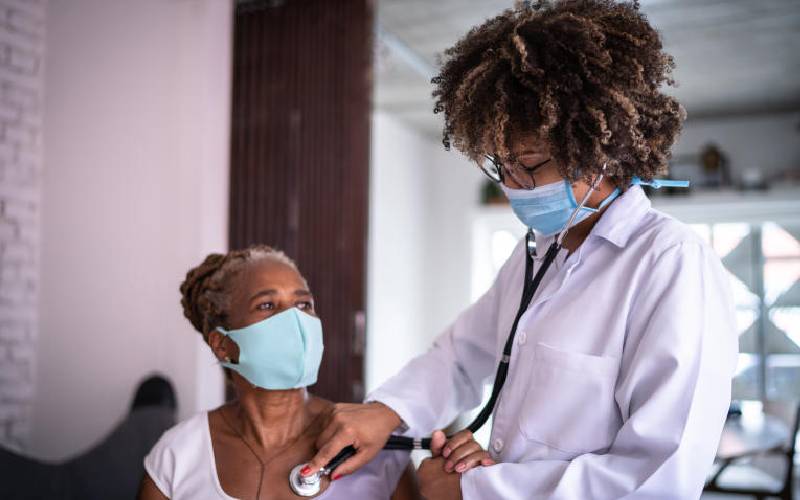
The main causes of diagnostic errors are lack of a systems approach to ensure diagnostic safety and quality. [Courtesy]
Diagnostic errors are common causes of wrong treatment, delayed treatment and poor health outcomes. But what are the issues surrounding diagnostic errors?