×
The Standard e-Paper
Smart Minds Choose Us
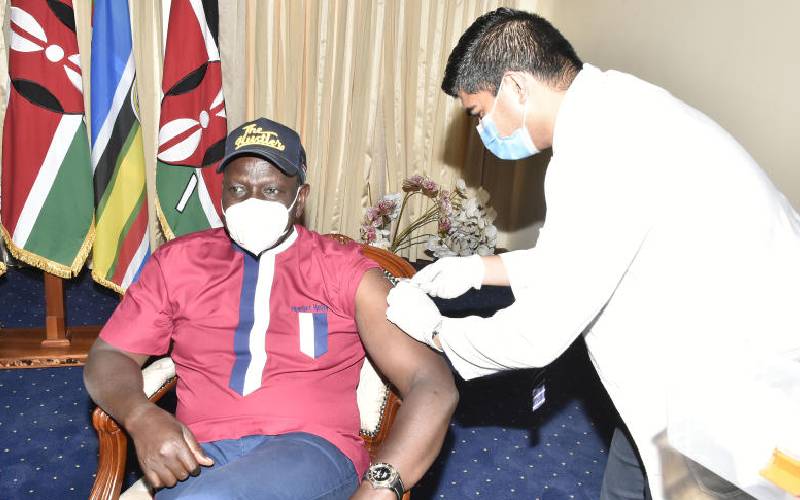
Deputy President William Ruto receives COVID-19 vaccine at his Karen office in Nairobi County on March 30, 2021.[DPPS]
Deputy President William Ruto has said he was not invited to the State House to receive the Oxford/AstraZeneca vaccine for Covid-19.