×
The Standard e-Paper
Kenya’s Boldest Voice
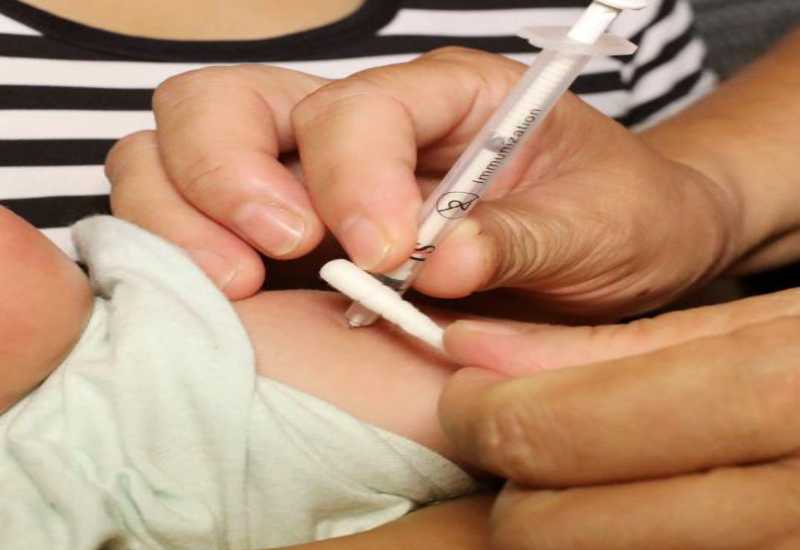
A child receives a vaccination shot at a hospital in Rongan in China's southern Guangxi region on July 23, 2018. [Photo/AFP]
Chinese Premier Li Keqiang has vowed stern action over the latest safety scare to hit the country’s pharmaceutical industry. This comes as a mounting scandal over a rabies vaccine sent drug stocks tumbling.